List of serious adverse reactions
List of serious adverse reactions encoded in MedDRA
According to the Art. 7 of the Ordinance on pharmacovigilance (Official Gazette, No. 83/13), HALMED publishes the list of adverse reactions considered to be serious, but not unambiguously determined according to the seriousness criteria stipulated in Article 7 and 8 of the Ordinance on pharmacovigilance (Official Gazette, No. 83/13) due to harmonisation of reporting the seriousness of individual adverse reactions.
The list of adverse reactions encoded in MedDRA is based on the list of serious adverse reactions of CIOMS V Working Group and the EMA's list of serious adverse reactions based on the "Important Medical Events" list (IME) issued by MSSO. These adverse reactions are categorised as "medically serious conditions". The marketing authorisation holder is required to proceed with the adverse reactions listed here as serious ones and to report them to HALMED, accordingly.
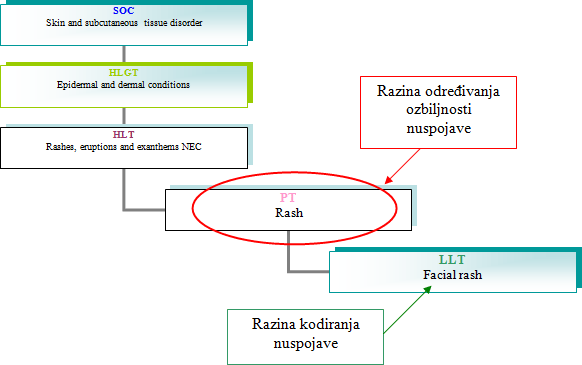
The list is based on the PT level of MedDRA coding that is in the most of the cases used for searching databases and in analysis. After an adverse reaction has been encoded in LLT based on the verbatim, the superimposed categories are automatically allocated.
Every adverse reaction at the PT level that is listed has a label (seriousness criteria):
- IME list - adverse reactions that according to the IME lsit must be encoded as serious (CS = "core serious")
- CIOMS V - adverse reactions that according to the CIOMS group must be encoded as serious.
The novelty related to the IME list is, that according to a decision of EudraVigilance Expert Working Group, it does not contain notions labelled as ES = "extended serious".
List of serious adverse reactions / IME list based on MedDRA updated version 28.1 (.xls)





